Takaki Miyata, MD., Ph.D.
Professor, Department of Anatomy and Cell Biology,
Nagoya University Graduate School of Medicine
Selected Publications
Miyata T
「Neocortical Neurogenesis in Development and Evolution」(Wiley)(Wieland E. Huttner Ed.)(Chapter 7 Mechanical and physical interactions involving neocortical progenitor cells)
Hattori Y, Kato D, Murayama F, Koike S, Asai H, Yamasaki A, Naito Y, Kawaguchi A, Konishi H, Prinz M, Masuda T, Wake H, Miyata T. CD206+ macrophages transventricularly infiltrate the early embryonic cerebral wall to differentiate into microglia.
Cell Rep., 2023.
DOI:10.1016/j.celrep.2023.1120
Tsujikawa K, Saito K, Nagasaka A, Miyata T. Developmentally interdependent stretcher-compressor relationship between the embryonic brain and the surrounding scalp in the preosteogenic head.
Developmental Dynamics, 08 Jan 2022.
DOI: https://doi.org/10.1002/dvdy.451
Nagasaka A, Miyata T. Comparison of the Mechanical Properties Between the Convex and Concave Inner/Apical Surfaces of the Developing Cerebrum.
Front. Cell Dev. Biol., 23 July 2021; 9.702068
DOI: 10.3389/fcell.2021.702068. eCollection 2021.
Hattori Y, Naito Y, Tsugawa Y, Nonaka S, Wake H, Nagasawa T, Kawaguchi A, Miyata T. Transient microglial absence assists postmigratory neurons in proper differentiation.
Nat. Commun. 2020 Apr 2; 11, 1631.
DOI: 10.1038/s41467-020-15409-3
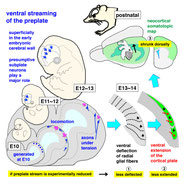
Saito K, Okamoto M, Watanabe Y, Noguchi N, Nagasaka A, Nishina Y, Shinoda T, Sakakibara A, Miyata T. Dorsal-to-Ventral Cortical Expansion Is Physically Primed by Ventral Streaming of Early Embryonic Preplate Neurons.
Cell Rep 2019 Nov 5; 29(6):1555-1567.
DOI: 10.1016/j.celrep.2019.09.075.
Yuto Watanabe, Takumi Kawaue, Takaki Miyata. Differentiating cells mechanically limit progenitor cells’ interkinetic nuclear migration to secure apical cytogenesis. Development., dev.162883 (2018).
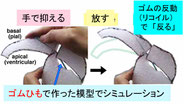
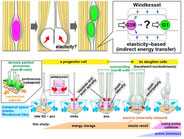
Shinoda T, Nagasaka A, Inoue Y, Higuchi R, Minami Y, Kato K, Suzuki M, Kondo T, Kawaue T, Saito K, Ueno N, Fukazawa Y, Nagayama M, Miura T, Adachi T, Miyata T. Elasticity-based boosting of neuroepithelial nucleokinesis via indirect energy transfer from mother to daughter. PLoS Biol., 16(4): e2004426. (2018)
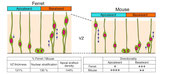
Nagasaka A, Shinoda T, Kawaue T, Suzuki T, Nagayama K, Matsumoto T, Ueno N, Kawaguchi A and Miyata T. Differences in the mechanical properties of the developing cerebral cortical proliferative zone between mice and ferrets at both the tissue and single-cell levels. Front. Cell Dev. Biol. 2016, 4:139, DOI: 10.3389/fcell.2016.00139
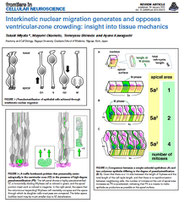
Miyata, T., Okamoto, M., Shinoda, T., Kawaguchi, A. Interkinetic nuclear migration generates and opposes ventricular-zone crowding: insights into tissue mechanics.
Front Cell Neurosci. 2015 Jan 28;8:473. doi: 10.3389/fncel.2014.00473.
Okamoto, M., Namba, T., Shinoda, T., Kondo, T., Watanabe, T., Inoue, Y., Takeuchi, K., Enomoto, Y., Ota, K., Oda, K., Wada, Y., Sagou, K., Saito, K., Sakakibara, A., Kawaguchi, A., Nakajima, K., Adachi, T., Fujimori, T., Ueda, M. Hayashi, S., Kaibuchi, K., Miyata, T. TAG-1–assisted progenitor elongation streamlines nuclear migration to optimize subapical crowding. Nat. Neurosci.,16: 1556-1566 (2013)
Miyata, T., Ono Y, Okamoto M, Masaoka M, Sakakibara A, Kawaguchi A, Hashimoto M, Ogawa M. Migration, early axonogenesis, and Reelin-dependent layer-forming behavior of early/posterior-born Purkinje cells in the developing mouse lateral cerebellum. Neural Dev. 5, 23 (2010)
Ochiai, W., Nakatani, S., Takahara, T., Kainuma, M., Masaoka, M., Minobe, S., Namihira, M., Nakashima, K., Sakakibara, A., Ogawa, M., Miyata, T.: Periventricular Notch activation and asymmetric Ngn2 and Tbr2 expression in pair-generated neocortical daughter cells. Mol. Cell. Neurosci. 40, 225-233 (2009)
Miyata, T.: Development of three-dimensional architecture of the neuroepithelium: Role of pseudostratification and cellular 'community'. Dev. Growth Differ. 50, S105-S112 (2008)
Miyata, T., and Ogawa, M.: Twisting of neocortical progenitor cells underlies a spring-like mechanism for daughter cell migration. Curr.Biol. 17, 146-151 (2007)
Miyata, T., Kawaguchi, A., Saito, K., Kawano, M., Muto, T., and Ogawa, M.: Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 131, 3133-3145 (2004)
Miyata, T., Kawaguchi, A., Okano, H., and Ogawa, M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31, 727-741 (2001)
Miyata, T., Maeda, T., and Lee, J.E.: NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes Dev. 13, 1647-1652 (1999)
Miyata, T., Nakajima, K., Mikoshiba, K., and Ogawa, M.: Regulation of Purkinje cell alignment by Reelin as revealed with CR-50 antibody. J. Neurosci. 17, 3599-3609 (1997)
Ogawa, M., Miyata, T., Nakajima, K., Yagyu, K., Ikenaka, K., Yamamoto, H., and Mikoshiba, K.: The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron 14, 899-912 (1995)
C.V. & Research History
Birth: 1963 (Kochi, Japan)
2004/1- present:
Professor, Department of Anatomy and Cell Biology, Nagoya University Graduate School of Medicine, Japan
1999/11-2003/12:
Research Scientist, Laboratory for Cell Culture Development (Dr. Masaharu Ogawa's Lab), Brain Science Institute, RIKEN, Japan
1998/10-1999/10:
Research Associate, Department of Neuroanatomy (Dr. Hideyuki Okano's Lab), Osaka University Graduate School of Medicine, Japan
1997/4-1998/9:
Postdoctoral Fellow, Department of Molecular, Cellular, and Developmental Biology (Dr. Jacqueline Lee's Lab), University of Colorado at Boulder, Colorado, USA
1994/4-1997/3:
Postdoctoral Fellow, Department of Molecular Neurobiology (Dr. Katsuhiko Mikoshiba's Lab), Institute for Medical Science, University of Tokyo, & Tsukuba Life Science Center, RIKEN Japan (stayed in Kochi to further collaborate with Dr. Masaharu Ogawa)
1990/4-1994/3:
Graduate Student, Department of Physiology, Kochi Medical School, Japan (supervised by Drs. Masaharu Ogawa & Akihiko Irimajiri)
1988/5-1990/3:
Department of Otolaryngology and Head and Neck Surgery (Prof. Haruo Saito), Kochi Medical School Hospital
After working as an otolaryngologist (Ear-Nose-Throat doctor) for two years, I decided to join the research field of Neural Development. Several years before this decision, i.e. when I was a medical student, I had already been interested in (and inspired by) the behaviors of cells undergoing their developmental processes in culture, which were shown by Dr. Masaharu Ogawa who was teaching physiology and also studying the “transdifferentiation” of adrenal chromaffin cells into neurons upon treatment with nerve growth factor (Nature 307, 66, 1984). Watching living cells as sincerely as possible, as if they were my patients, is the heart of my research style, which was established during my graduate school period (Develop. Growth & Differ. 36, 319, 1994).
As a postdoc, I worked for the following two major projects: (1) Reelin. A Japanese research group that I belonged (Ogawa and Mikoshiba team) tried to identify a certain molecule missing in the reeler mouse mutant. We immunized adult reeler mice with homogenates of normal embryonic brain tissues, expecting that the immunized reeler mice might provide us a molecular probe (antibody) that would recognize a gene product playing important roles in the formation of the developing brain. That strategy nicely worked (Neuron 14, 899, 1995), and it soon turned out that our antibody indeed recognized the product of reelin that was cloned by Curran’s group in the same year (Nature 374, 719, 1995). I screened anti-Reelin hybridomas and carried out blocking experiments by applying anti-Reelin to a various kinds of three-dimensional culture systems (J. Neurosci. 17, 3599, 1997).
(2) NeuroD. I worked under Dr. Jacqueline Lee who found that this bHLH factor is important for neuronal differentiation in Xenopus (Science 268, 836, 1995). I analyzed the phenotype of neuroD-KO mice. These KO mice suffered from hyperglycemia due to failed differentiation of pancreatic b cells but this diabetic phenotype was rescued by putting neuroD under the control of insulin promoter. Using this pancreatic-rescued version of the neuroD-KO mice, I found that granule neurons in the developing cerebellar cortex and those in the hippocampal dendate gyrus are missing. The reason of absence of granule cells was turned out to be cell death (Genes & Dev. 13, 1647, 1999).
Then, I have been working on neural progenitor cells. After making efforts to prospectively identify neural stem cells (Mol. Cell. Neurosci. 17, 259, 2001) in Dr. Hideyuki Okano’s lab, I moved to RIKEN BSI (reunited with Dr. Ogawa) and established a slice culture technique to observe 3D behaviors of neocortical progenitor cells. My report (Neuron 14, 899, 2001), together with very relevant reports including Dr. Kriegstein lab’s work (Nature 409, 714, 2001), evidenced that “radial glial cells” are proliferative and neurogenic, not a quiescent guidepost (for migratory neurons) as believed earlier. It also demonstrated that radial glial fibers can be inherited by neurons and used for neuronal migration.
Slice culture is also useful to observe non-stem-like, intermediate progenitor cells dividing at non-surface positions in the developing cerebral wall (Development 131, 3133, 2004) and to carry out mechanical and pharmacological experiments (Curr. Biol. 17, 146, 2007). Based on visualization methods for single cells in slice culture, we now live monitor all cells in slice by using transgenic mice carrying reporters like H2B-mCherry or lyn-Venus. This new approach allows us to understand how neighboring cells interact physically and how interkinetic nuclear migration is controlled mechanically (Nat. Neurosci. 2013).