英語論文
Asai, H., Ono, M., Miyata, T. and Hattori.
FlashTag-mediated Labeling for Intraventricular Macrophages in the Embryonic Brain.
doi: 10.21769/BioProtoc.5166.
Shimamura T, Miyata T. Quantitative in toto live imaging analysis of apical nuclear migration in the mouse telencephalic neuroepithelium
doi: 10.1111/dgd.12949.
Tsujikawa K, Muramatsu R, Miyata T. CSF pressure in fetal mice in utero: External factors pressurize the intraventricular space
doi: https://doi.org/10.1101/2024.09.08.611845
Murayama F, Asai H, Patra A. K, Wake H, Miyata T, Hattori Y. A novel preparation for histological analyses of intraventricular macrophages in the embryonic brain.
Dev Growth Differ., 2024 Jun 19; 66(5):329-337.
doi: 10.1111/dgd.12935
Miyata T 「Neocortical Neurogenesis in Development and Evolution」(Wiley)(Wieland E. Huttner Ed.)(Chapter 7 Mechanical and physical interactions involving neocortical progenitor cells)2023 Aug
https://doi.org/10.1002/9781119860914.ch7
Hattori Y. The multifaceted roles of embryonic microglia in the developing brain.
Front. Cell. Neurosci., 2023 May 12:17:988952.
DOI: 10.3389/fncel.2023.988952
Hattori Y, Kato D, Murayama F, Koike S, Asai H, Yamasaki A, Naito Y, Kawaguchi A, Konishi H, Prinz M, Masuda T, Wake H, Miyata T. CD206+ macrophages transventricularly infiltrate the early embryonic cerebral wall to differentiate into microglia.
Cell Rep., 2023 Feb 28;42(2):112092.
DOI:10.1016/j.celrep.2023.1120
Oguma T, Takigawa-Imamura H, Shinoda T, Ogura S, Uemura A, Miyata T, Maini PK, and Miura T. Analyzing the effect of cell rearrangement on Delta-Notch pattern formation.
Phys Rev E. 2023 Jun;107(6-1):064404.
DOI: 10.1103/PhysRevE.107.064404
Katsuta H, Okuda S, Nagayama K, Machiyama H, Kidoaki S, Kato M, Sokabe M, Miyata T, Hirata H. Actin crosslinking by α-actinin averts viscous dissipation of myosin force transmission in stress fibers.
iScience. 2023 Feb 1;26(3):106090.
DOI: 10.1016/j.isci.2023.106090
Saito D, Tadokoro R, Nagasaka A, Yoshino D, Teramoto T, Mizumoto K, Funamoto K, Kidokoro H, Miyata T, Tamura K, Takahashi Y. Stiffness of primordial germ cells is required for their extravasation in avian embryos.
iScience. 2022 Nov 18;25(12):105629.
DOI: 10.1016/j.isci.2022.105629
Hattori Y. The multiple roles of pericytes in vascular formation and microglial functions in the brain.
Life (Basel). 2022 Nov 9;12(11):1835.
DOI:10.3390/life12111835
Hattori Y. The microglia-blood vessel interactions in the developing brain.
Neurosci Res. 2023 Feb:187:58-66.
DOI: 10.1016/j.neures.2022.09.006
Wang S, Fu Y, Miyata T, Matsumoto S, Shinoda T, Itoh K, Harada A, Hirotsune S, Jin M. Functional cooperation of α-synuclein and tau is essential for proper corticogenesis.
J Neurosci., 2022 Sep 14;42(37):7031-7046.
DOI: 10.1523/JNEUROSCI.0396-22.2022
Ogura Y, Sahashi K, Hirunagi T, Iida M, Miyata T, Katsuno M. Mid1 is associated with androgen-dependent axonal vulnerability of motor neurons in spinal and bulbar muscular atrophy.
Cell Death Dis., 2022 Jul 13;13(7):601.
DOI: 10.1038/s41419-022-05001-6.
Tsujikawa K, Hamanaka K, Riku Y, Hattori Y, Hara N, Iguchi Y, Ishigaki S, Hashizume A, Miyatake S, Mitsuhashi S, Miyazaki Y, Kataoka M, Jiayi L, Yasui K, Kuru S, Koike H, Kobayashi K, Sahara N, Ozaki N, Yoshida M, Kakita A, Saito Y, Iwasaki Y, Miyashita A, Iwatsubo T. Japanese Alzheimer’s Disease Neuroimaging Initiative (J-ADNI); Ikeuchi T, Japanese Longitudinal Biomarker Study in PSP and CBD (JALPAC) Consortium; Miyata T, Sobue G, Matsumoto N, Sahashi K, Katsuno M. Actin-binding protein filamin-A drives tau aggregation and contributes to progressive supranuclear palsy pathology.
Sci Adv., 2022 May 27;8(21).
DOI: 10.1126/sciadv.abm5029
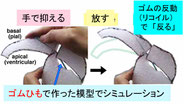
Tsujikawa K, Saito K, Nagasaka A, Miyata T. Developmentally interdependent stretcher-compressor relationship between the embryonic brain and the surrounding scalp in the preosteogenic head.
Developmental Dynamics, 2022 Jul;251(7):1107-1122.
DOI: https://doi.org/10.1002/dvdy.451
Hattori Y, Itoh H, Tsugawa Y, Nishida Y, Kurata K, Uemura A, Miyata T. Embryonic pericytes promote microglial homeostasis and their effects on neural progenitors in the developing cerebral cortex.
The Journal of Neuroscience, 2022 Jan 19;42(3):362-376.
DOI: 10.1523/JNEUROSCI.1201-21.2021
Hattori Y. The behavior and functions of embryonic microglia.
Anatomical Science International, 2022 Jan;97(1):1-14.
DOI: 10.1007/s12565-021-00631-w,
Li Chenmin, Konishi H, Nishiwaki K, Sato K, Miyata T, Kiyama H. A mouse model of microglia-specific ablation in the embryonic central nervous system.
Neuroscience Research, 2021 Jun 23; 173:54-61.
DOI: 10.1016/j.neures.2021.06.002
Nagasaka A, Miyata T. Comparison of the Mechanical Properties Between the Convex and Concave Inner/Apical Surfaces of the Developing Cerebrum.
Front. Cell Dev. Biol., 23 July 2021; 9.702068
DOI: 10.3389/fcell.2021.702068. eCollection 2021.
Tsujikawa K, Saito K, Nagasaka A, Miyata T. Mechanical collaboration between the embryonic brain and the surrounding scalp tissues.
DOI: https://doi.org/10.1101/2021.05.05.442865
Hattori Y, Naito Y, Tsugawa Y, Nonaka S, Wake H, Nagasawa T, Kawaguchi A, Miyata T. Transient microglial absence assists postmigratory neurons in proper differentiation.
Nat. Commun. 2020 Apr 2; 11, 1631.
DOI: 10.1038/s41467-020-15409-3
Kawasoe R, Shinoda T, Hattori Y, Nakagawa M, Pham TQ, Tanaka Y, Sagou K, Saito K, Katsuki S, Kotani T, Sano A, Fujimori T, Miyata T. Two-photon microscopic observation of cell-production dynamics in the developing mammalian neocortex in utero.
Dev Growth Differ. 2020 January 14;62:118-128.
DOI: 10.1111/dgd.12648.
▶PubMed
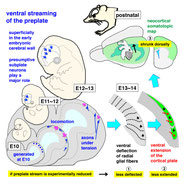
Saito K, Okamoto M, Watanabe Y, Noguchi N, Nagasaka A, Nishina Y, Shinoda T, Sakakibara A, Miyata T. Dorsal-to-Ventral Cortical Expansion Is Physically Primed by Ventral Streaming of Early Embryonic Preplate Neurons.
Cell Rep 2019 Nov 5; 29(6):1555-1567.
DOI: 10.1016/j.celrep.2019.09.075.
Mizutani Y, Kobayashi H, Iida T, Asai N, Masamune A, Hara A, Esaki N, Ushida K, Mii S, Shiraki Y, Ando K, Weng L, Ishihara S, Ponik SM, Conklin MH, Haga H, Nagasaka A, Miyata T, Matsuyama M, Kobayashi T, Fujii T, Yamada S, Yamaguchi J, Wang T, Woods SL, Worthley D, Shimamura T, Fujishiro M, Hirooka Y, Takahashi M, and Enomoto A. Meflin-positive cancer-associated fibroblasts inhibit pancreatic carcinogenesis. Cancer Res 2019 Oct 15;79(20):5367-5381.
DOI: 10.1158/0008-5472
Hara A, Kobayashi H, Asai N, Shigeyoshi S, Higuchi T, Kato K, Okumura T, Bando YK, Takefuji M, Mizutani Y, Miyai Y, Saito S, Maruyama S, Maeda K, Ouchi N, Nagasaka A, Miyata T, Mii S, Kioka N, Worthley DL, Murohara T, Takahashi M, Enomoto A. Roles of the Mesenchymal Stromal/Stem Cell Marker Meflin in Cardiac Tissue Repair and the Development of Diastolic Dysfunction. Circ Res. 2019 Aug 2;125(4):414-430
DOI: 10.1161/CIRCRESAHA.119.314806.
Kawaue T, Shitamukai A, Nagasaka A, Tsunekawa Y, Shinoda T, Saito K, Terada R, Bilgic M, Miyata T, Matsuzaki F & Kawaguchi A. Lzts1 controls both neuronal delamination and outer radial glial-like cell generation during mammalian cerebral development.
Nat Commun 2019 Jun 25;10(1):2780.
DOI: 10.1038/s41467-019-10730-y.
Hattori Y, Miyata T. Embryonic neocortical microglia express Toll-like receptor 9 and respond to plasmid DNA injected into the ventricle: technical considerations regarding microglial distribution in electroporated brain walls. eNeuro 2018 Nov 16; ENEURO.0312-18.2018.
DOI: https://doi.org/10.1523/ENEURO.0312-18.2018
Kawaguchi A. Temporal patterning of neocortical progenitor cells: how do they know the right time? Neuroscience Research 2018 Sep 15; 138:3-11.
DOI: 10.1016/j.neures.2018.09.004
Hattori Y, Miyata T. Microglia extensively survey the developing cortex via the CXCL12/CXCR4 system to help neural progenitors to acquire differentiated properties. Genes Cells. 2018 Aug 24. [Epub ahead of print]
DOI: 10.1111/gtc.12632.
Pham TQ, Kawaue T, Hoshi T, Tanaka Y, Miyata T, Sano A. Role of extrinsic mechanical force in the development of the RA-I tactile mechanoreceptor. Sci Rep. 2018 Jul 23;8(1):11085.
DOI: 10.1038/s41598-018-29390-x.
Watanabe Y, Kawaue T, Miyata T. Differentiating cells mechanically limit progenitor cells’ interkinetic nuclear migration to secure apical cytogenesis. Development. June 26 2018, dev.162883.
DOI: 10.1242/dev.162883
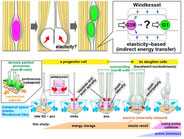
Shinoda T, Nagasaka A, Inoue Y, Higuchi R, Minami Y, Kato K, Suzuki M, Kondo T, Kawaue T, Saito K, Ueno N, Fukazawa Y, Nagayama M, Miura T, Adachi T, Miyata T. Elasticity-based boosting of neuroepithelial nucleokinesis via indirect energy transfer from mother to daughter. PLOS Biol. April 20 2018, 16(4): e2004426.
DOI: 10.1371/journal.pbio.2004426
Ohtaka-Maruyama C, Okamoto M, Endo Kentaro, Oshima M, Kaneko N, Yura K, Okado H, Miyata T, Maeda N. Synaptic transmission from subplate neurons controls radial migration of neocortical neurons. Science. Apr 20 2018, 360(6386): 313-317.
DOI: 10.1126/science.aar2866
Jinnou H, Sawada M, Kawase K, Kaneko N, Herranz-Pérez V, Miyamoto T, Kawaue T, Miyata T, Tabata Y, Akaike T, García-Verdugo JM, Ajioka I, Saitoh S, Sawamoto K. Radial Glial Fibers Promote Neuronal Migration and Functional Recovery after Neonatal Brain Injury. Cell Stem Cell. Jan 04 2018, 22(1): 128-137.e9.
DOI: http://dx.doi.org/10.1016/j.stem.2017.11.005
Saito K, Kawasoe R, Sasaki H, Kawaguchi A, Miyata T. Neural Progenitor Cells Undergoing Yap/Tead-Mediated Enhanced Self-Renewal Form Heterotopias More Easily in the Diencephalon than in the Telencephalon. Neurochemical Research. Jan 2018, 43(1): 1-10.
DOI: 10.1007/s11064-017-2390-x
Delaunay D, Kawaguchi A, Dehay C and Matsuzaki F. Division modes and physical asymmetry in cerebral cortex progenitors. Curr Opin Neurobiol. 2017, 42:75–83. DOI:10.1016/j.conb.2016.11.009
Matsunaga Y, Noda M, Murakawa H, Hayashi K, Nagasaka A, Inoue S, Miyata T, Miura T, Kubo K, and Nakajima K. Reelin transiently promotes N-cadherin-dependent neuronal adhesion during mouse cortical development. Proc.Natl.Acad.Sci.U.S.A. 2017,114(8):2048-2053. DOI:10.1073/pnas.1615215114
Ogura S, Kurata K, Hattori Y, Takase H, Ishiguro-Oonuma T, Hwang Y, Ahn S, Park I, Ikeda W, Kusuhara S, Fukushima Y, Nara H, Sakai H, Fujiwara T, Matsushita J, Ema M, Hirashima M, Minami T, Shibuya M, Takakura N, Kim P, Miyata T, Ogura Y, Uemura A. Sustained inflammation after pericyte depletion induces irreversible blood-retina barrier breakdown. JCI Insight. 2017,2(3):e90905, DOI:10.1172/jci.insight.90905.
Jin M, Pomp O, Shinoda T, Toba, Torisawa T, Furuta K, Oiwa K, Yasunaga T, Kitagawa D, Matsumura S, Miyata T, Tan TT, Reversade B and Hirotsune S. Katanin p80, NuMA and cytoplasmic dynein cooperate to control microtubule dynamics. Sci Rep. 2017, 7:39902, DOI: 10.1038/srep39902.
Nagasaka A, Shinoda T, Kawaue T, Suzuki T, Nagayama K, Matsumoto T, Ueno N, Kawaguchi A and Miyata T. Differences in the mechanical properties of the developing cerebral cortical proliferative zone between mice and ferrets at both the tissue and single-cell levels. Front. Cell Dev. Biol. 2016, 4:139, DOI: 10.3389/fcell.2016.00139
Kawaguchi A and Matsuzaki F. Cell cycle–arrested cells know the right time. Cell Cycle 2016,15:20, 2683-2684, DOI: 10.1080/15384101.2016.1204857
Pham TQ, Hoshi T, Tanaka Y, Sano A, Kawaue T, Miyata T. Two-Photon imaging of DiO-labelled Meissner corpuscle in living mouse's fingertip. IEEE Trans Haptics. 2016, 9:4, 483-491, DOI: 10.1109/TOH.2016.2574718
Itoh, Y., Higuchi, M., Oishi, K., Kishi, Y., Okazaki, T., Sakai, H., Miyata, T., Nakajima, K. and Gotoh, Y. PDK1–Akt pathway regulates radial neuronal migration and microtubules in the developing mouse neocortex. PNAS.113 E2955-E2964, 2016 doi: 10.1073/pnas.1516321113
Okamoto, M., Miyata, T., Konno, D., Ueda, H. R., Kasukawa, T., Hashimoto, M., Matsuzaki, F., Kawaguchi, A. Cell-cycle-independent transitions in temporal identity of mammalian neural progenitor cells. Nat Commun. 2016 Apr 20;7:11349. doi:10.1038/ncomms11349.
Katsunuma, S., Honda, H., Shinoda, T., Ishimoto, Y., Miyata, T., Kiyonari, H., Abe, T., Nibu, K., Takai, Y., Togashi, H. Synergistic action of nectins and cadherins generates the mosaic cellular pattern of the olfactory epithelium. J. Cell. Biol. 2016 Feb 29; 212, 561-575
Leto, K., Arancillo, M., Becker, E. B. E., Buffo, A., Chiang, C., Ding, B., Dobyns, W. B., Dusart, I., Haldipur, P., Hatten, M. E., Hoshino, M., Joyner, A. L., Kano, M., Kilpatrick, D. L., Koibuchi, N., Marion, S., Martinez, S., Millen, K. J., Millner, T. O., Miyata, T., Parmigiani, E., Schilling, K., Sekerkova, G., Sillitoe, R. V., Sotelo, C., Uesaka, N., Wefers, A., Wingate, R. J. T., Hawkes, R. Consensus paper: Cerebellar development. Cerebellum. 06 October, 2015 DOI 10.1007/s12311-015-0724-2
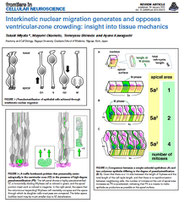
Miyata, T., Okamoto, M., Shinoda, T., Kawaguchi, A. Interkinetic nuclear migration generates and opposes ventricular-zone crowding: insights into tissue mechanics.
Front Cell Neurosci. 2015 Jan 28;8:473. doi: 10.3389/fncel.2014.00473.
Okamoto, M., Shinoda, T., Kawaue, T., Nagasaka, A., Miyata, T. Ferret-mouse differences in interkinetic nuclear migration and cellular densification in the neocortical ventricular zone. Neurosci. Res. 83, 25-32, 2014
Kawaue T, Sagou K, Kiyonari H, Ota K, Okamoto M, Shinoda T, Kawaguchi A, Miyata T. Neurogenin2-d4Venus and Gadd45g-d4Venus transgenic mice: Visualizing mitotic and migratory behaviors of cells committed to the neuronal lineage in the developing mammalian brain. Dev Growth Differ. 56, 293-304, 2014
Hashimoto, M., Hata, A., Miyata, T., Hirase, H. Programmable wireless light-emitting diode stimulator for chronic stimulation of optogenetic molecules in freely moving mice. Neurophoton. 1(1), 011002 (May 28, 2014). doi:10.1117/1.NPh.1.1.011002
Namba, T., Kibe, Y., Funahashi, Y., Nakamuta, S., Takano, T., Ueno, T., Shimada, A., Kozawa, S., Okamoto, M., Shimoda, Y., Oda, K., Wada, Y., Masuda, T., Sakakibara, A., Igarashi, M., Miyata, T., Faivre-Sarrailh, C., Takeuchi, K., Kaibuchi, K. Pioneering axons regulate neuronal polarization in the devveloping cerebral cortex. Neuron 81, 814-829, 2014
Sakakibara, A.(corresponding author), Sato, T., Ando, R., Noguchi, N., Masaoka, M., Miyata, T. Dynamics of centrosome translocation and microtubule organization in neocortical neurons during distinct modes of polarization. Cereb. Cortex 24, 1301-1310, 2014 (doi:10.1093/cercor/bhs411)
Ageta-Ishihara, N., Miyata, T., Ohshima, C., Watanabe, M., Sato, Y., Hamamura, Y., Higashijima, T., Mazitschek, R., Bito, H., Kinoshita, M. Septins promote dendrite and axon development by negatively regulating microtubule stability via HDAC6-mediated deacetylation. Nat. Commun. 4: 2532, 2013, DOI: 10.1038/ncomms3532
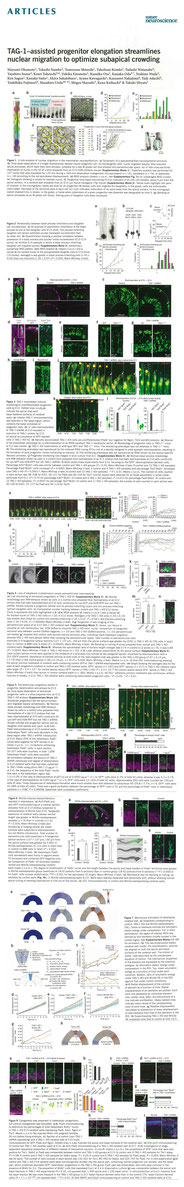
Okamoto, M., Namba, T., Shinoda, T., Kondo, T., Watanabe, T., Inoue, Y., Takeuchi, K., Enomoto, Y., Ota, K., Oda, K., Wada, Y., Sagou, K., Saito, K., Sakakibara, A., Kawaguchi, A., Nakajima, K., Adachi, T., Fujimori, T., Ueda, M. Hayashi, S., Kaibuchi, K., Miyata, T. TAG-1–assisted progenitor elongation streamlines nuclear migration to optimize subapical crowding. Nat. Neurosci., 16: 1556-1566 (2013) DOI: 10.1038/nn.3525
Sakakibara, A., Ando, R., Sapir, T., Tanaka, T. Microtubule dynamics in neuronal morphogenesis. (2013) Open Biol. 3:130061 DOI: 10.1098/rsob.130061
Sapir, T., Levy, T., Sakakibara, A., Rabinkov, A., Miyata, T., Reiner, O. Shootin1 acts in concert with KIF20B to promote polarization of migrating neurons. (2013) J. Neurosci. 33:11932-11948 DOI: 10.1523/JNEUROSCI.5425-12.2013
Wu, J , Liu, L , Matsuda, T , Zao, Y, Rebane, A , Drobizhev, M , Chang, Y-F , Araki, S , Arai, Y , March, K , Thomas, HE, Sagou, K , Miyata, T, Nagai, T , Li, W-H , and Campbell, RE Improved orange and red Ca2+ indicators and photophysical considerations for optogenetic applications. ACS Chem Neurosci. 2013 Jun 19;4(6):963-72. doi: 10.1021/cn400012b. Epub 2013 Mar 19.
Xie, M.-J., Yagi, H., Kuroda, K., Wang, C.-C., Komada, M., Zhao, H., Sakakibara, A., Miyata, T. Nagata, K., Iguchi, T., Sato, M. WAVE2-Abi2 complex controls growth cone activity and regulates the multipolar-bipolar transition as well as the initiation of glia-guided migration. Cereb. Cortex 23:1410-1423 (2013) (doi: 10.1093/cercor/bhs123)
Pérez-Martínez, F.J., Luque-Río, A., Sakakibara, A., Hattori, M., Miyata,
T., Luque, J. M. Reelin-dependent ApoER2 downregulation uncouples newborn neurons from progenitor cells. Biol. Open 1:1258-1263 (2012)
Nakamuta, S., Funahashi, Y., Namba, T., Arimura, N., Picciotto, M.R., Tokumitsu, H., Soderling, T.R., Sakakibara, A., Miyata, T., Kamiguchi, H., Kaibuchi, K. Local application of neurotrophins specifies axons through inositol 1,4,5-trisphosphate, calcium, and ca2+/calmodulin-dependent protein kinases. Sci. Signal. 4(199):ra76 (2011)
Natsume, S., Kato, T., Kinjo, S., Enomoto, A., Toda, H., Shimato, S., Ohka, F., Motomura, K., Kondo, Y., Miyata, T., Takahashi, M., Wakabayashi, T. Girdin maintains the stemness of glioblastoma stem cells. Oncogene 31, 2715-2724 (2011)
Miyata, T., Ono Y, Okamoto M, Masaoka M, Sakakibara A, Kawaguchi A, Hashimoto M, Ogawa M. Migration, early axonogenesis, and Reelin-dependent layer-forming behavior of early/posterior-born Purkinje cells in the developing mouse lateral cerebellum. Neural Dev. 5, 23 (2010)
Miyata, T., Kawaguchi, D., Kawaguchi, A., Gotoh, Y. Mechanisms that regulate the number of neurons during mouse neocortical development. Curr. Opin. Neurobiol. 20, 22-28 (2010)
Kato TM, Kawaguchi A, Kosodo Y, Niwa H, *Matsuzaki F.
Lunatic fringe potentiates Notch signaling in the developing brain. Mol Cell Neurosci. 45, 12-25, 2010
Uchida T, Baba A, Perez-Martinez FJ, Hibi T, Miyata T, Luque JM, Nakajima K, Hattori M. Downregulation of functional Reelin receptors in projection neurons implies that primary Reelin action occurs at early/premigratory stages. J Neurosci. 29:10653-62 (2009)
Saito K, Dubreuil V, Arai Y, Wilsch-Brauninger M, Schwudke D, Saher G, Miyata T, Breier G, Thiele C, Shevchenko A, Nave KA, Huttner WB. Ablation of cholesterol biosynthesis in neural stem cells increases their VEGF expression and angiogenesis but causes neuron apoptosis. Proc Natl Acad Sci U S A 106(20):8350-5 (2009)
Minobe, S., Sakakibara, A., Ohdachi, T., Kanda, R., Kimura, M., Nakatani, S., Tadokoro, R., Ochiai, W., Nishizawa, Y., Mizoguchi, A., Kawauchi, T., Miyata, T.: Rac is involved in the interkinetic nuclear migration of cortical progenitor cells. Neurosci. Res. 63, 294-301 (2009)
Ochiai, W.,* Nakatani, S.,* Takahara, T., Kainuma, M., Masaoka, M., Minobe, S., Namihira, M., Nakashima, K., Sakakibara, A., Ogawa, M., Miyata, T.: Periventricular Notch activation and asymmetric Ngn2 and Tbr2 expression in pair-generated neocortical daughter cells. Mol. Cell. Neurosci. 40, 225-233 (2009) (*Equal contribution)
Yoon, K.-J., Koo, B.-K., Jeong, H.-W., Ghim, J., Kwon, M.-C., Moon, J.-S., Miyata, T., Kong, Y.-Y.: Mind bomb 1-experssing intermediate progenitors generate Notch signaling to maintain radial glial cells. Neuron 58, 519-531 (2008)
Sunabori, T., Tokunaga, A., Nagai, T., Sawamoto, K., Okabe, M., Miyawaki, A., Matsuzaki, Y., Miyata, T., Okano, H.: Cell-cycle-specific nestin expression coordinates with morphological changes in embryonic cortical neural progenitors. J. Cell Sci. 121, 1204-1212 (2008)
Koyasu T, Kondo M, Miyata K, Ueno S, Miyata T, Nishizawa Y, Terasaki H. Photopic electroretinograms of mGluR6-deficient mice. Curr Eye Res. 33, 91-99 (2008)
Miyata, T.: Development of three-dimensional architecture of the neuroepithelium: Role of pseudostratification and cellular 'community'. Dev. Growth Differ. 50, S105-S112 (2008)
Sakaue-Sawano, A., Kurokawa, H., Morimura, T., Hanyu, A., Hama, H., Osawa, H., Kashiwagi, S., Fukami, K., Miyata, T., Miyoshi, H., Imamura, T., Ogawa, M., Masai, H. and Miyawaki, A.: Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132, 487-498 (2008).
Konno, D., Shioi, G., Shitamukai, A., Mori, A., Kiyonari, H., Miyata, T. and Matsuzaki, F.: Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat. Cell Biol. 10, 93-101 (2008)
Nishizawa, Y., Imafuku, H., Saito, K., Kanda, R., Kimura, M., Minobe, S., Miyazaki, F., Kawakatsu, S., Masaoka, M., Ogawa, M. and Miyata, T.: Survey of the morphogenetic dynamics of the ventricular surface of the developing mouse cortex. Dev. Dyn. 236, 3061-3070 (2007)
Tamai, H., Shinohara, H., Miyata, T., Saito, K., Nishizawa, Y., Nomura, T. and Osumi, N.: Pax6 transcription factor regulates interkinetic nuclear movement in cortical progenitor cells via centrosomal stabilization. Genes Cells 12, 983-996 (2007)
Miyata, T.: Morphology and mechanics of daughter cells "delaminating" from the ventricular zone of the developing neocortex. Cell Adh. Migr. 1, 99-101(2007)
Miyata, T., and Ogawa, M.: Twisting of neocortical progenitor cells underlies a spring-like mechanism for daughter cell migration. Curr.Biol. 17, 146-151 (2007)
Ochiai, W., Minobe, S., Ogawa, M., Miyata, T.: Transformation of pin-like ventricular zone cells into cortical neurons. Neurosci. Res. 57, 326-329 (2007)
Miyata, T.: Asymmetric cell division during brain morphogenesis. Prog. Mol. Subcell. Biol. 452, 121-142 (2007)
Hirai, S., Cui, DF., Miyata, T., Ogawa, M., Kiyonari, H., Suda, Y., Aizawa, S., Banda, Y. and Ohno, S.: The c-Jun N-terminal kinase activator dual leucine zipper kinase regulates axon growth and neuronal migration in the developing cerebral cortex. J. Neurosci. 26, 11992-12002 (2006)
Imai, F., Hirai, S., Akimoto, K., Koyama, H., Miyata, T., Ogawa, M., Noguchi, S., Sasaoka, T., Noda, T., and Ohno, S.: Inactivation of aPKCλ results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development 133, 1735-1744 (2006)
Mutoh, T., Miyata, T., Kashiwagi, S., Miyawaki, A., and Ogawa, M.: Dynamic behavior of individual cells in developing organotypic brain slices revealed by the photoconvertable protein Kaede. Exp. Neurol. 200, 430-437 (2006)
Naruse, M., Nakahira, E., Miyata, T., Hitoshi, S., Ikenaka, K., and Bansai, R.: Induction of oligodendrocyte progenitors in dorsal forebrain by intraventricular microinjection of FGF-2. Dev. Biol. 60, 1084-1100 (2006)
Zou, P., Muramatsu, H., Miyata, T., and Muramatsu, T.: Midkine, a heparin-binding growth factor, is expressed in neural precursor cells and promotes their growth. J. Neurochem. 99, 1470-1479 (2006)
Miyata, T., Saito, K., Nishizawa, Y., Murayama, A., Masaoka, M., and Ogawa, M.: Modern slice culture for direct observation of production and migration of brain neurons. Nagoya J. Med. Sci. 67, 65-70 (2005)
Ueno S, Kondo M, Miyata K, Hirai T, Miyata T, Usukura J, Nishizawa Y, Miyake Y. Physiological function of S-cone system is not enhanced in rd7 mice. Exp Eye Res. 81, 751-758 (2005)
Uematsu J, Nishizawa Y, Hirako Y, Kitamura K, Usukura J, Miyata T, Owaribe K. Both type-I hemidesmosomes and adherens-type junctions contribute to the cell-substratum adhesion system in myoepithelial cells. Eur J Cell Biol. 84, 407-415 (2005)
Kawaguchi, A., Ogawa, M., Saito, K., Matsuzaki, F., Okano, H., and Miyata, T.: Differential expression of Pax6 and Ngn2 between pair-generatged cortical neurons. J. Neurosci. Res. 78, 784-795 (2004)
Miyata, T., Kawaguchi, A., Saito, K., Kawano, M., Muto, T., and Ogawa, M.: Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 131, 3133-3145 (2004)
<名古屋大着任以前の宮田の論文>
Saito, K., Kawaguchi, A., Kashiwagi, S., Yasugi, S., Ogawa, M., and Miyata, T.: Morphological asymmetry in dividing retinal progenitor cells. Develop. Growth & Differ. 45, 219-229 (2003)
Shinozaki, K., Miyagi, T., Yoshida, M., Miyata, T., Ogawa, M., Aizawa, S., and Suda, Y.: Absence of Cajal-Retzius cells and subplate neurons associated with defects of tangential migration from ganglionic eminence in Emx1/2 double mutant cerebral cortex. Development 129, 3479-3492 (2002)
Miyata, T., Kawaguchi, A., Saito, K., Kuramochi, H., and Ogawa, M.: Visualization of cell cycling by an improvement in slice culture methods. J. Neurosci. Res. 69, 861-868 (2002)
Ogawa Y, Sawamoto K, Miyata,T., Miyao S, Watanabe M, Nakamura M, Bregman BS, Koike M, Uchiyama Y, Toyama Y, Okano H.: Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J. Neurosci. Res. 69, 925-933 (2002).
Takasawa K, Kitagawa K, Yagita Y, Sasaki T, Tanaka S, Matsushita K, Ohstuki T, Miyata T, Okano H, Hori M, Matsumoto M. Increased proliferation of neural progenitor cells but reduced survival of newborn cells in the contralateral hippocampus after focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 22, 299-307 (2002).
Yagita Y, Kitagawa K, Sasaki T, Miyata, T., Okano H, Hori M, Matsumoto M. Differential expression of Musashi1 and nestin in the adult rat hippocampus after ischemia. J. Neurosci. Res. 69, 750-756 (2002).
Yamazaki, Y., Makino, H., Hamaguchi-Hamada, K., Hamada, S., Sugino, H., Kawase, E., Miyata, T., Ogawa, M., Yanagimachi. R., and Yagi, T.: Assessment of the developmental totipotency of neural cells in the cerebral cortex of mouse embryo by nuclear transfer. Proc. Natl. Acad. Sci. USA S 98, 14022-14026 (2001)
Miyata, T., Kawaguchi, A., Okano, H., and Ogawa, M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31, 727-741 (2001)
Kawaguchi, A., Miyata, T., Sawamoto, K., Takashita, N., Murayama, A., Akamatsu, W., Ogawa, M., Okabe, M., Tano, Y., Goldman, S.A., and Okano, H. Nestin-EGFP mice: visualization of the self-renewal and multipotency of CNS stem cells. Mol. Cell. Neurosci. 17, 259-273 (2001)
Yagita, Y., Kitagawa, K., Otsuki, T., Kuwabara, K., Mabuchi, T., Miyata, T., Okano, H., Hori, M., and Matsumoto, M.: Proliferation of neuronal progenitor cells and increased neurogenesis in the ischemic adult rat hippocampus. Stroke 32, 1890-1896 (2001)
Kaneko, Y., Sakakibara, S., Imai, T., Suzuki, A., Nakamura, Y., Sawamoto, K., Ogawa, Y., Toyama, Y., Miyata, T., and Okano, H.: Musashi1: an evolutionally conserved marker for CNS progenitor cells including neural stem cells. Dev. Neurosci. 22, 139-153 (2000)
Nakamura, Y., Sakakibara, S., Miyata, T., Ogawa, M., Shimazaki, T., Weiss, S., Kageyama, R., and Okano, H.: The bHLH gene Hes1 as a repressor of neuronal commitment of the CNS stem cells. J. Neurosci. 20, 283-293 (2000)
Ohtani, T., Ishihara, K., Atsumi, T., Nishida, K., Keneko, Y., Miyata, T., Itoh, S., Narimatsu, M., Maeda, H., Fukada, T., Itoh, M., Okano, H., Hibi, T., and Hirano, T.: Dissection of signaling cascade through gp130 in vivo: Reciprocal roles for STAT3-and SHP2-mediated signals in cytokine and immunoglobulin production. Immunity 12, 95-105 (2000)
Miyata, T., Maeda, T., and Lee, J.E.: NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes Dev. 13, 1647-1652 (1999)
Miyata, T., Nakajima, K., Mikoshiba, K., and Ogawa, M.: Regulation of Purkinje cell alignment by Reelin as revealed with CR-50 antibody. J. Neurosci. 17, 3599-3609 (1997)
Miyata, T., Nakajima, K., Mikoshiba, K., and Ogawa, M.: Distinct arrangement patterns of Purkinje cells between normal and reeler mice are reproduced in cerebellar explants. Dev. Neurosci. 19, 124 (1997)
Del Rio, J., Heimrich, B., Borrell, V., Froster, E., Drakew, A., Alcantara, S., Nakajima, K., Miyata, T., Ogawa, M., Mikoshiba, K., Derer, P., Frotscher, M., and Soriano, E.: A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature 385, 70-74 (1997)
Nakajima, K., Mikoshiba, K., Miyata, T., Kudo, C., and Ogawa, M.: Disruption of hippocampal development in vivo by CR-50 mAb against Reelin. Proc. Natl. Acad. Sci. USA 94, 8196-8201 (1997)
De Vergeyck, V., Nakajima, K., Lambert de Rouvroit, C., Naerhuyzen, B., Goffinet, A. M., Miyata, T., Ogawa, M., and Mikoshiba, K.: A truncated Reelin protein is produced but not secreted in the “Orleans” reeler mutation. Mol. Brain Res. 50, 85-90 (1997)
D’Arcangelo, G., Nakajima, K., Miyata, T., Ogawa, M., Mikoshiba, K., Curran, T.: Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J. Neurosci. 17, 23-31 (1997)
Yoneshima, H., Nagata, E., Matsumoto, M., Yamada, M., Nakajima, K., Miyata, T., Ogawa, M., and Mikoshiba, K.: A novel neurological mutant mouse, yotari, which exhibits reeler-like phenotype but expresses CR-50 antigen/Reelin. Neurosci. Res. 29, 217-223 (1997)
Miyata, T., Nakajima, K., Aruga, J., Takahashi, S., Ikenaka, K., Mikoshiba, K, and Ogawa, M.: Distribution of a reeler gene-related antigen in the developing cerebellum: an immunohistochemical study with an allogeneic antibody CR-50 on normal and reeler mice. J Comp. Neurol. 372, 215-228 (1996)
Sakakibara, S., Okano, H., Imai, T., Hamaguchi, K., Aruga, J., Nakajima, K., Nagata, T., Kurihara, Y., Uesugi, S., Miyata, T., Ogawa, M., and Mikoshiba, K.: Mouse-musashi-1, a neural RNA-binding protein highly enriched in the mammalian CNS stem cell. Dev. Biol. 176, 230-242 (1996)
Takahashi, S., Yamamoto, H., Matsuda, Z., Ogawa, M., Yagyu, K., Taniguchi, T., Miyata, T., Koda, H., Higuchi, T., Okutani, F., and Fujimoto, S.: Identification of two highly homologous presynaptic proteins distinctly localized at the dendritic and somatic synapses. FEBS letters 368, 455-460 (1995)
Ogawa, M., Miyata, T., Nakajima, K., Yagyu, K., Ikenaka, K., Yamamoto, H., and Mikoshiba, K.: The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron 14, 899-912 (1995)
Miyata, T., and Ogawa, M.: Developmental potentials of early telencephalic neuroepithelial cells: a study with microexplant culture. Dev. Growth & Differ. 36, 319-331 (1994)